Strukturní biologie signálních proteinů
Ph.D./M.Sc. positions available for motivated students
PhD/M.Sc. project: The role of calcium and 14-3-3 in the regulation of human ubiquitin ligase Nedd4-2
Laboratory website: http://www.biomed.cas.cz/d312/
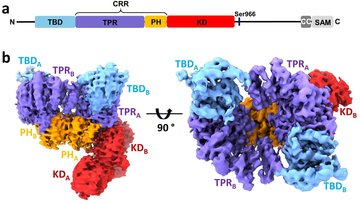
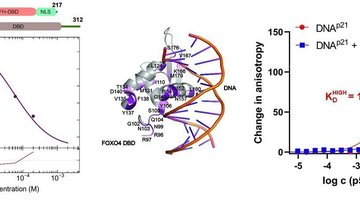
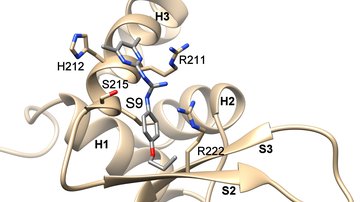
The main goal of this project is to elucidate the structural basis of the regulation of human neuronal precursor cell-expressed developmentally down-regulated 4-2 (Nedd4-2) ubiquitin ligase through calcium cations and 14-3-3 proteins. Nedd4-2 is a key regulator of Na+ homeostasis as it ubiquitinates various channels and membrane transporters, including the epithelial sodium channel ENaC. Dysregulation of Nedd4-2 leads to a number of pathologies, including electrolytic imbalance, respiratory distress, hypertension, and kidney diseases. It was shown, that calcium cations dramatically increase the ligase activity of Nedd4-2, which further requires binding of the C2 domain to the membrane to stabilize the active conformation of Nedd4-2. In contrast, 14-3-3 binding inhibits Nedd4-2 activity. However, many aspects of these regulatory mechanisms remain unclear. The proposed research will allow us to understand the processes leading to the activation and inhibition of Nedd4-2 activity. The main methods will be site-directed mutagenesis, analytical ultracentrifugation, NMR, H/D exchange and chemical cross-linking coupled with mass spectrometry, SAXS, cryoelectron microscopy, fluorescence spectroscopy, lipid binding assay and ubiquitination assay. The research will be performed in close cooperation with the Faculty of Science, Charles University.
References:
- Joshi R, Pohl P, Strachotova D, Herman P, Obsil T*, Obsilova V* (2022) Nedd4-2 binding to 14-3-3 modulates the accessibility of its catalytic site and WW domains. Biophys J. 121(7):1299-1311. https://pubmed.ncbi.nlm.nih.gov/35189105/
- Pohl P, Joshi R, Petrvalska O, Obsil T* and Obsilova V* (2021) 14-3-3-protein regulates Nedd4-2 by modulating interactions between HECT and WW domains. Commun. Biol. 4(1):899. https://www.nature.com/articles/s42003-021-02419-0
- Lentini Santo, D., Petrvalska O., Obsilova V., Ottmann C. and Obsil T. (2020) Stabilization of protein-protein interactions between CaMKK2 and 14-3-3 by fusicoccins. ACS Chem Biol. 15(11):3060-3071. https://pubmed.ncbi.nlm.nih.gov/33146997/
- Hagenbuchner, J, Obsilova, V, Obexer, P, Obsil, T*, Ausserlechner, MJ* (2021). Discovery of Small Compounds that Target FOXO Transcription Factors and Modulate their Transcriptional Activity and Physiological Function. Book chapter in “New Innovations in Chemistry and Biochemistry” Vol. 4, pp 41-54. https://doi.org/10.9734/bpi/nicb/v4/14349D
- Kalabova D+, Filandr F+, Alblova M, Petrvalska O, Horvath M, Man P, Obsil T*, Obsilova V* (2020) 14-3-3 protein binding blocks the dimerization interface of caspase-2. FEBS J. 287:3494-3510. https://pubmed.ncbi.nlm.nih.gov/31961068/
- Hagenbuchner, J+, Obsilova, V+, Kaserer T+, Kaiser N, Rass B, Psenakova K, Docekal V, Alblova M, Kohoutova K, Schuster D, Aneichyk T, Vesely J, Obexer P, Obsil T*, Ausserlechner MJ* (2019) Modulating FOXO3 transcriptional activity by small, DBD-binding molecules. Elife 8, pii: e48876. https://pubmed.ncbi.nlm.nih.gov/31789593/
- Smidova A+, Alblova M+, Kalabova D+, Psenakova K, Rosulek M, Herman P, Obsil T*, Obsilova V* (2018) 14-3-3 protein masks the nuclear localization sequence of caspase-2. FEBS J. 285:4196-4213. https://pubmed.ncbi.nlm.nih.gov/30281929/
- Alblova M, Smidova A, Docekal V, Vesely J, Herman P, Obsilova V*, Obsil T* (2017) Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1. Proc Natl Acad Sci U S A. 114:E9811-E9820. https://pubmed.ncbi.nlm.nih.gov/29087344/
Project Supervisors (email): Veronika Obsilova, Ph.D. (veronika.obsilova@fgu.cas.cz), prof. Tomas Obsil, Ph.D. (obsil@natur.cuni.cz)
Candidate´s profile (requirements): Successful candidate should enjoy working in the field of structural biology/biophysics/physical chemistry, be curious and has the courage to try out new things. Required is M.Sc. degree or those expecting to obtain their degree this year (fields: Chemistry, Biochemistry, Molecular Biology or similar). The knowledge of molecular biology, protein expression and purification is a strong advantage. Good knowledge of English is required. We offer: friendly, motivating environment, part-time/full-time contract for 3 years with the possibility of renewal, 5 weeks of vacation, subsidized lunch.