Mice and genetic scissors help Czech researchers find treatment for the happy child syndrome
Researchers from the Czech Centre for Phenogenomics (The Institute of Molecular Genetics of the Czech Academy of Sciences in the BIOCEV Centre) have been developing mouse models that help to discover the causes of human diseases and enable testing of new therapies. These include cancer, diabetes, infertility, but also rare diseases. 300 million people worldwide suffer from rare, with approximately 600 000 in the Czech Republic. These diseases are most often manifested shortly after birth.
Oliver was born a healthy boy. At the age of about four months, his parents noticed a slight delay in development. When he was a year old, genetic tests revealed Angelman syndrome, an incurable rare disease.
Frequent smiles and good mood gave this disease the unofficial name of the “happy child” syndrome. Children who suffer from it are referred to as “angels”. However, there is another side to the diagnosis. This involves decreased intellect, problems with motor skills and sleep, epileptic seizures. “Angel” children never speak, they communicate only with simple pictograms. For the rest of their lives, they are dependent on the care of others.

“I will never forget the moment I first learned what Angelman syndrome was. My heart stopped then. I felt like I would never be able to breathe again! They told us that our baby had an incurable disease and would be completely dependent on us for the rest of his life,” says Oliver's mother Lenka Hajgajda, describing her first feelings after the diagnosis.
In 2018, the Hajgajdas founded the Association of Gene Therapy (ASGENT). A non-profit organization whose purpose is to support research into rare diseases. It was the first time in the Czech Republic that patients themselves initiated basic research into a rare disease and approached researchers from the Czech Centre for Phenogenomics.
Orphan diseases
In Europe, a rare disease is defined as one that affects less than one person out of a 2000. More than 7000 rare diseases have been identified in the world. They are characterised by a wide variety of disorders and symptoms that vary from disease to disease and patient to patient even in people suffering from the same disease.
Due to the low prevalence of each disease, there is a lack of medical specialisation and detailed knowledge of each disease. Research in this area is also limited, not only because of the low economic returns. Despite the large total number of rare diseases, patients become “orphans” of health systems, as diagnosis, treatment and access to research results are often inadequate.
“72% of rare diseases have a genetic basis. This is where the Czech Centre for Phenogenomics can help. We create genetically engineered mouse models with a non-functional gene. This allows to model a human disease,” says Radislav Sedláček, Director of the Centre.

Why do they use mice? Approximately 98% of mouse genes are similar to human genes. "There are some genes that do not have a particular impact directly on physiology but may play a role in the development of a disease. The function of other genes, which account to about one third, is so important that if we disable them, the embryo will not develop and the mouse will not be born at all. And precisely these genes often cause diseases and problems during the development of the foetus,” explains Radislav Sedláček.
Together with international partners, they are checking the function of all the genes that humans and mice have in common. If they succeed, they will create an encyclopaedia of gene functions, one of the most important milestones in biology and medicine.
“Only when we get to know exactly what individual genes do, will we be able to, figuratively speaking, put together whole sentences from words and then better understand what genes are needed for and how they contribute to the development of diseases,” says Radislav Sedláček.
Rare Diseases Research at the Czech Centre for Phenogenomics
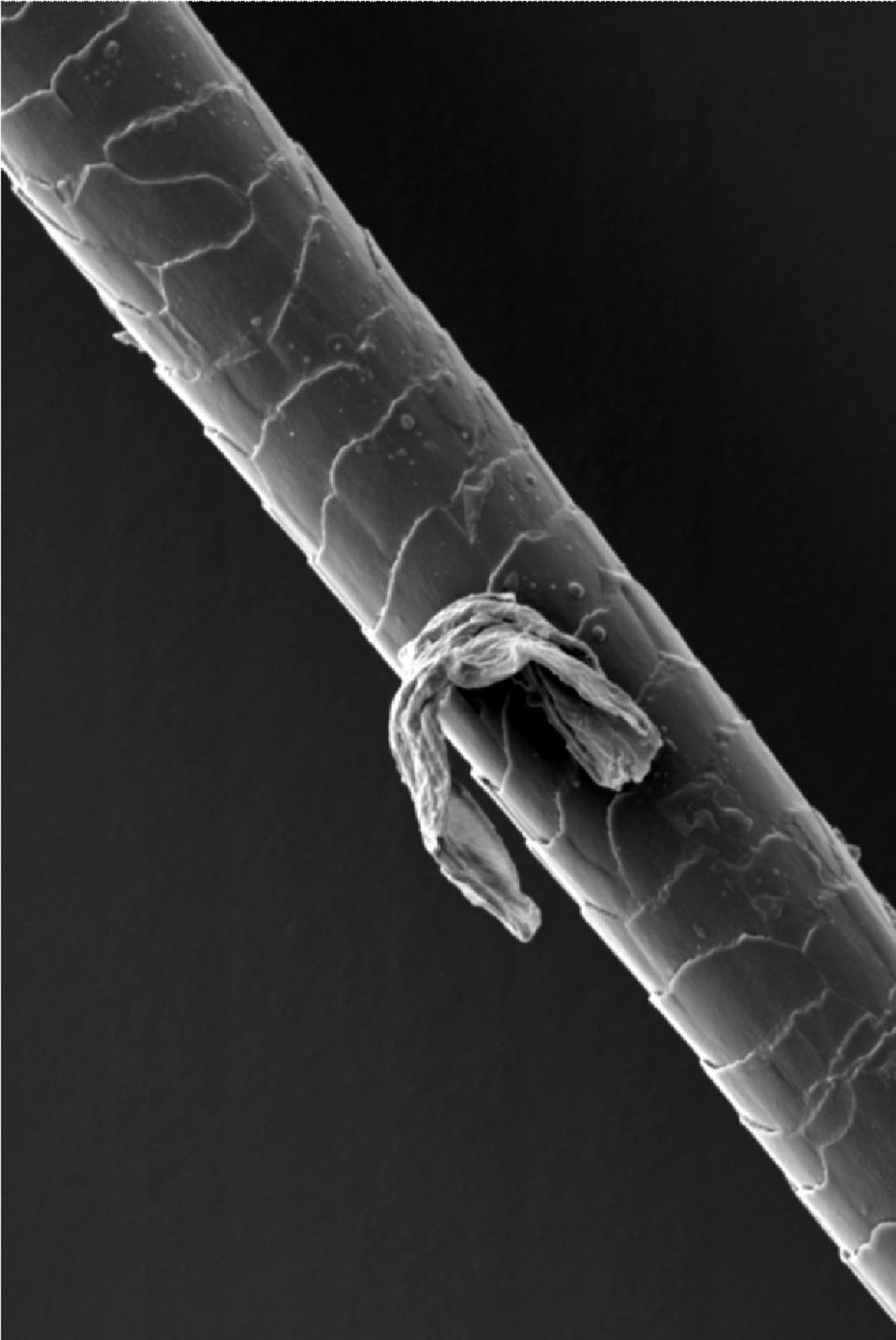
Netherton syndrome
- It manifests itself as a severe skin condition, allergies or asthma. It is sometimes referred to as bamboo hair syndrome because the hair of patients resembles fragile bamboo stalks with typical roots.
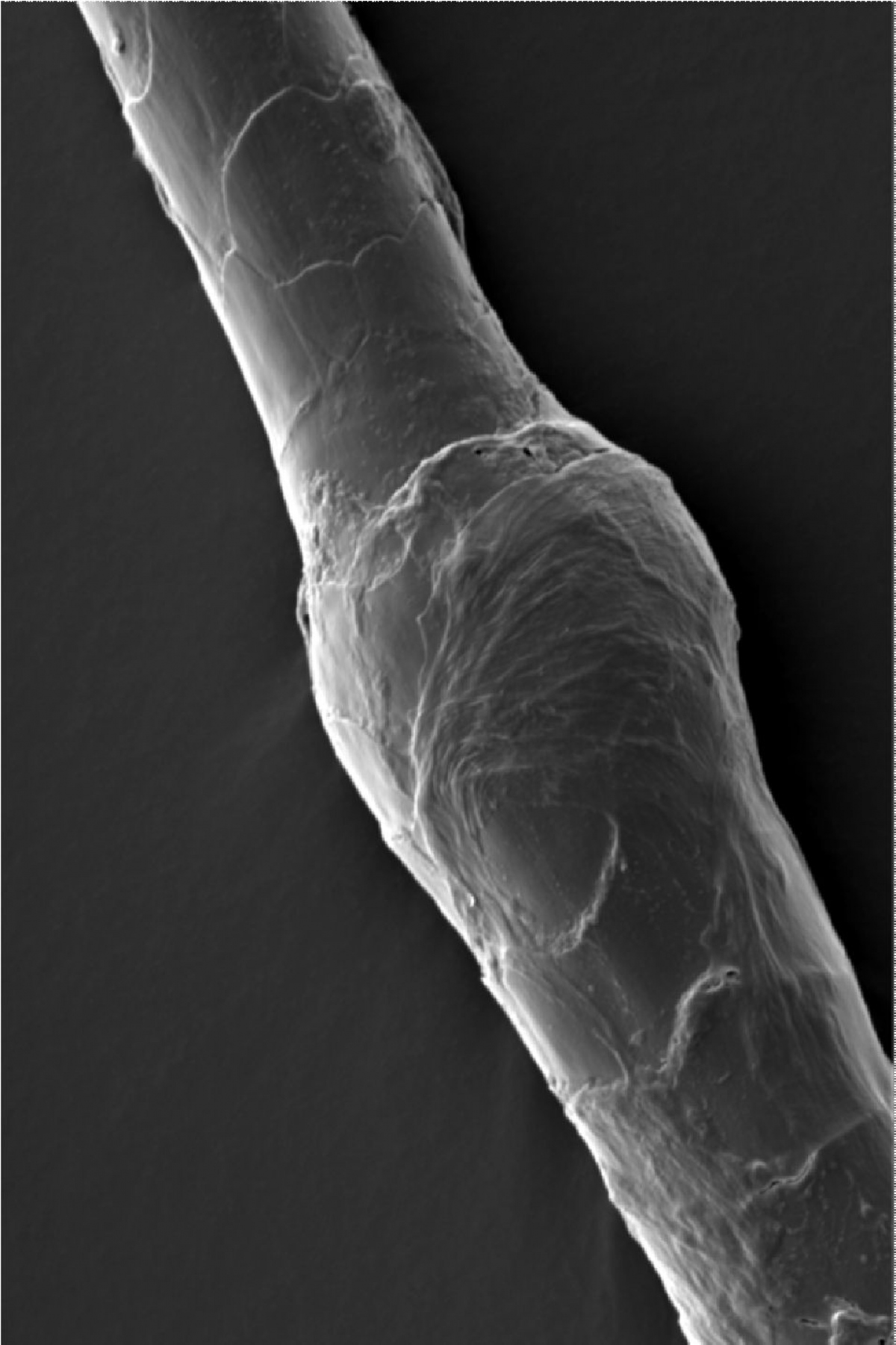
Angelman syndrome
- The syndrome is characterized by balance disorders, coordination, severe mental retardation, absence of speech, and sleep disorders. Typical features include hand flapping, puppet-like gait, fascination with water, and always smiling expression.
Prader-Willi syndrome
- It is characterized mainly by uncontrollable appetite, small stature, delayed puberty and mild intellectual disability. It is the most common genetic cause of life-threatening obesity in children.
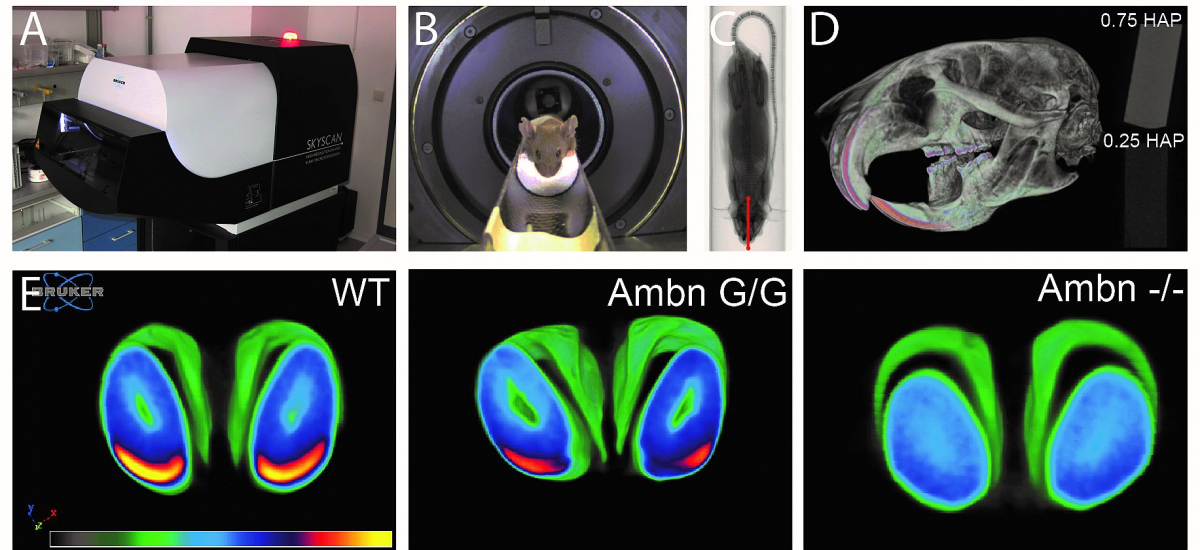
Amelogenesis imperfecta
- A disorder of tooth development causes teeth to be unusually small, have various defects and are prone to rapid wear and breakage.
B - Genetically engineered mice undergoing microCT scanning under anesthesia
C - Visualization of the mouse during scanning in the computer program; the red line shows the area selected for analysis
D - Image of the whole skull with the lower jaw in pseudocolours to better show the density of bone mineralisation
E - Cross-sectional image of the mouse incisors from microCT. The most mineralized part of the enamel is shown in yellow (a normal mouse was imaged). In genetically engineered mice, the second image shows the loss of the mineralized portion of the enamel and the third image shows the complete absence of enamel.
Osteogenesis imperfecta
- It is known as “brittle bone disease”. It is a group of genetic disorders that cause bones to break easily. Other symptoms include blue whites of the eyes, short stature, loose joints, hearing loss, breathing problems and dental problems.
Achondroplasia
- Characterised by dwarfism with short limbs.
Genetic scissors
When creating a mouse model, scientists most often use the “genetic scissors”, the CRISPR-Cas9 method, which was awarded the Nobel Prize in Chemistry in 2020. The method allows scientists to work with a specific stretch of DNA and repair the affected gene. The genetic modification occurs at the level of the single-cell mouse embryo and, as a result, the informative mutation is present in all adult cells.
With CRISPR-Cas9, the mouse gene can be changed or turned off completely. The model is then handed over to specialists who perform detailed physiological tests, such as in lungs, the cardiovascular system, vision, hearing, neurobehavioral, clinical biochemistry, immunology, metabolism or histopathology tests.

Together with bioinformaticians, they describe the model in detail. Each mouse will then undergo a complex series of diagnostic tests that will reveal how a particular gene works in the body, where its absence manifests itself and what effect it has on the eventual development of a disease.
"We also use this approach to clarify the origin of genetic rare diseases and to understand in detail the behaviour of cells with a complete absence of genes or modified genes that cause the disease,” explains Radislav Sedláček, adding: "In mice with Angelman syndrome (AS), we have been able to see how the effects of silenced genes can be influenced, and even mitigated. We believe that our research will also contribute to elucidating the role, function and regulation of the gene responsible for AS, as well as finding a way to compensate for its loss."
Czech mice for the entire world
The cost of creating a mouse model depends on the complexity of the gene and be as much as CZK 200 000. Subsequent basic phenotyping analysis starts at CZK 500 000 and can reach several million, especially for genes related to systemic diseases affecting multiple organs.
Mouse models also provide an immense help in the development and testing of new drugs and therapies. They are used in preclinical testing to test the safety and efficacy of new drugs. If preclinical testing is successful, clinical testing continues. The entire process takes an average of 7 years (1.5 years for preclinical testing and 5 years for clinical trials). The development of a new medicine from the start of research to market launch takes on average of 12 to 15 years.

“The mouse models created at our centre have a great reputation in the research and pharmaceutical community. We are in discussions with several partners from pharmaceutical companies who want to use our models to create or test revolutionary experimental therapies. It makes me very happy that our work is finding more and more applications for the development of treatments for often very serious diseases,” says Radislav Sedláček.
Download images and logos HERE
The Czech Centre for Phenogenomics (CCP) at the Institute of Molecular Genetics of the Czech Academy of Sciences is a large research infrastructure, uniquely combining genetic engineering, advanced phenotyping and cryoarchiving models. The CCP is strategically located in one place in the BIOCEV centre. Since January 2022, the CCP has been the coordinator of the research programme “GENE AND PRECISION THERAPY – A NEW HOPE IN THE TREATMENT OF HUMAN DISEASES” of the AV21 Strategy. www.phenogenomics.cz
The BIOCEV Centre is a joint research facility of six institutes of the Czech Academy of Sciences and two faculties of Charles University, located in Vestec near Prague. The aim of more than 500 researchers and students from all over the world is to gain a detailed understanding of organisms at the molecular level. Their findings are directed towards the research and development of new drugs and treatments for serious health problems such as cancer, diabetes, infertility and viral diseases. www.biocev.eu
The Institute of Molecular Genetics of the Czech Academy of Sciences (IMG) is a public research institution, part of the Czech Academy of Sciences, which conducts research in the field of molecular, structural and cell biology, immunology, functional genomics and bioinformatics. https://www.img.cas.cz