New findings on the structure of the protein kinase ASK1 - a key enzyme in the regulation of the cellular response to stress
Apoptosis signal-regulating kinase 1 (ASK1) is a crucial stress sensor, directing cells toward apoptosis, differentiation, and senescence via the p38 and JNK signaling pathways. ASK1 dysregulation has been associated with cancer and inflammatory, cardiovascular, and neurodegenerative diseases, among others. However, the molecular mechanism by which this important protein kinase is regulated is still unclear. In a work published in the journal eLife, scientific teams of Dr. Obsilova (IPHYS CAS) and prof. Obsil (Faculty of Science, Charles University and IPHYS CAS) characterized the structure of ASK1 and its interactions with thioredoxin at the molecular level. New knowledge about the structure and interactions of ASK1 may facilitate the development of new inhibitors for this enzyme, leading to a novel anti-inflammatory therapy.
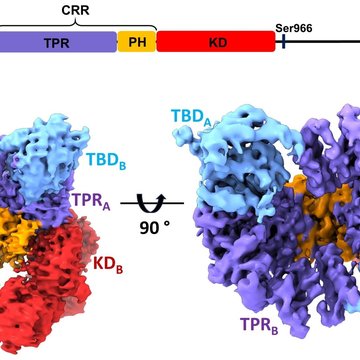
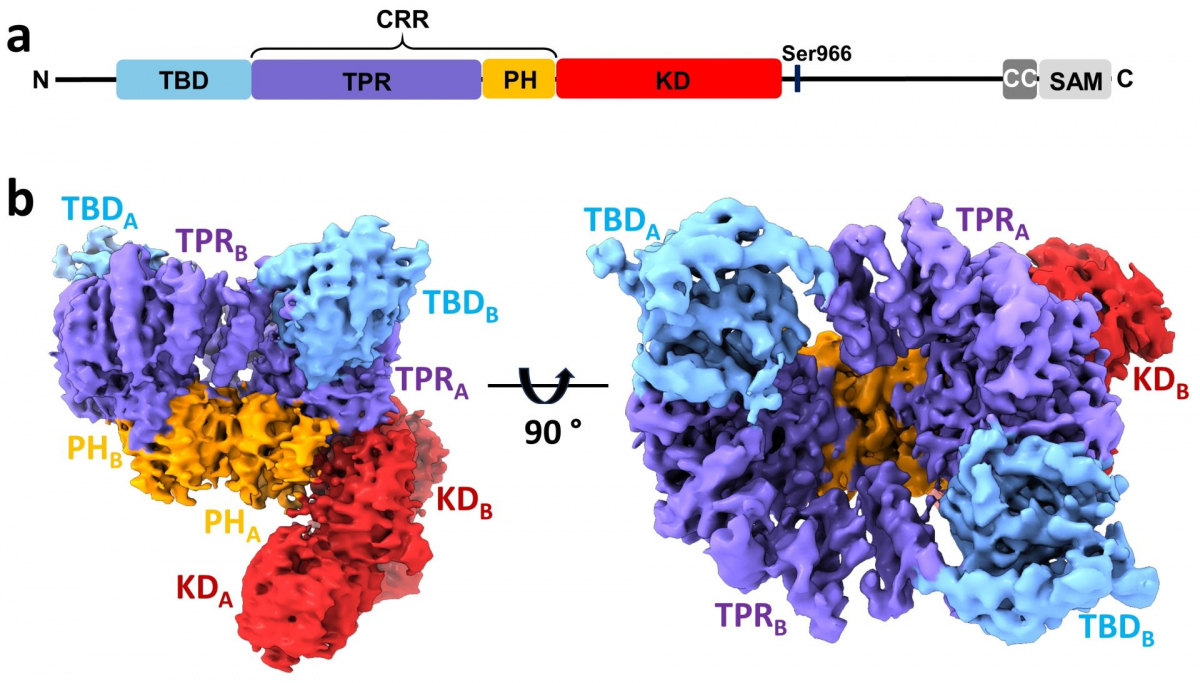
In this structural study, researchers performed a detailed characterization of ASK1 and its interactions with its binding partner thioredoxin (TRX1) using an integrated approach involving cryo-electron microscopy, hydrogen-deuterium exchange coupled to mass spectrometry, and analytical ultracentrifugation. ASK1 is a multi-domain protein kinase with a complex regulatory mechanism involving dimerization and interactions with several other proteins, including TRX1. The researchers studied the role of individual domains and mapped their interactions and importance for dimerization.
They discovered that the ASK1, when in the active state, forms a compact and asymmetric dimer, which enables extensive interdomain and interchain interactions that stabilize the active conformation of the kinase domain (Fig.). Through an interaction study, they further found that TRX1 acts as a negative allosteric effector of ASK1, modifying the structure and interactions of the individual domains of ASK1. Consequently, TRX1 reduces access to the activation segment of the kinase domain. Overall, this study not only clarifies the role of ASK1 dimerization and inter-domain contacts but also provides key mechanistic insights into its regulation, thereby highlighting the potential of ASK1 protein-protein interactions as targets for anti-inflammatory therapy.
Link to publication: Karolina Honzejkova, Dalibor Kosek, Veronika Obsilova, Tomas Obsil. The cryo-EM structure of ASK1 reveals an asymmetric architecture allosterically modulated by TRX1. eLife. 2024 Mar 27;13:RP95199. doi: 10.7554/eLife.95199.












