New results concerning a key complex regulating the Krebs cycle and oxidative phosphorylation
Mitochondria harbour two important pathways: the Krebs cycle and oxidative phosphorylation (OXPHOS). While Krebs cycle is a hub of catabolic and anabolic pathways essential for proper physiology and function of a cells, OXPHOS is the major source of cellular energy in the for of ATP. Thus, the two pathways are under strict control. They are connected with each other by mitochondrial respiratory complex II (CII), also referred to as succinate de-hydrogenase (SDH). CII is a protein complex, whose assembly mechanism has not been re-solved. In recent research, laboratory of Jiří Neužil (Institute of Biotechnology) together with groups of Gary Cecchini (University of California in San Francisco, CA, USA) and Tina Iverson (Vanderbilt University, Nashville, TN, USA, has successfully resolved the initial steps of CII assembly.
Mitochondrial respiratory CII is at the cross-roads of the Krebs cycle and OXPHOS, with an important regulatory role in majority of cell types, including cancer cells. Of note, it is frequently deregulated/mutated in certain types of cancer, including endocrine cancers (paraganglioma, pheochromocytoma), renal cancer and gastrointestinal cancer. CII includes four subunits, SDHA, SDHA, SDHC and SDHD, and its proper assembly requires four dedicated assembly factors, SDHAF1, SDHAF2, SDHAF3 and SDHAF4.
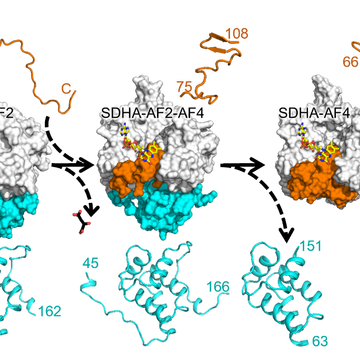
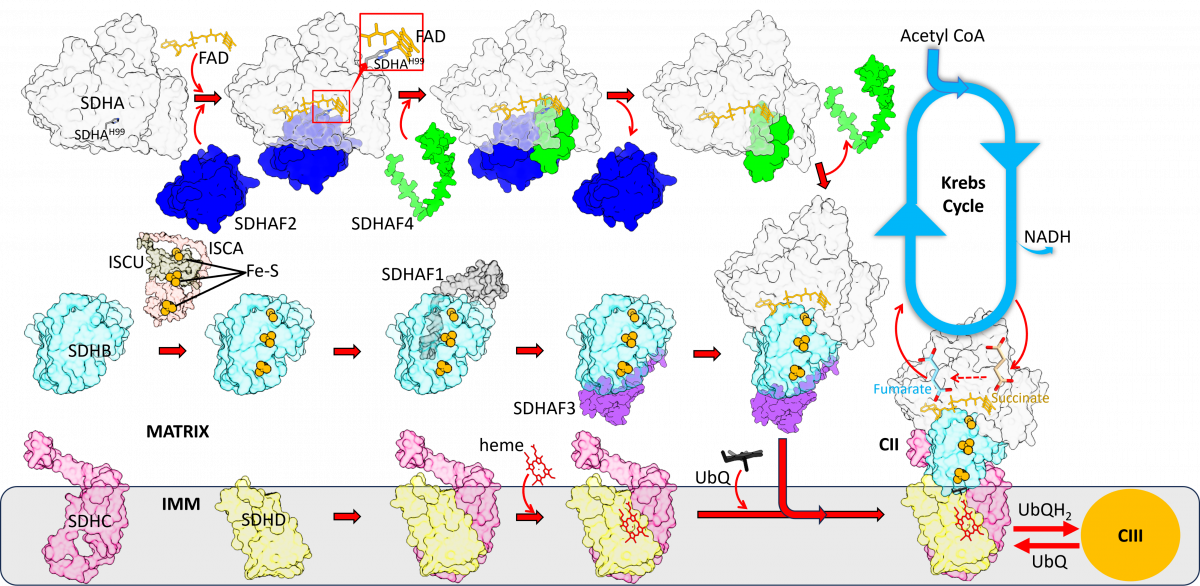
We now show that assembly of CII requires, in its first step, proper maturation of the catalytic subunit SDHA. The proposed pathway is shown in the above cartoon, starting with the holo-protein SDHA that requires SDHAF2 and a dicarboxylate to integrate a molecular of FAD. The metastable SDHA-SDHAF2 sub-assembly then recruits SDHAF4 that associates with SDHA, now containing FAD, to remove SDHAF2. This involves transient formation of the SDHA-SDHAF2-SDHAF4 ternary metastable sub-assembly followed by formation of the SDHA-SDHAF4 sub-assembly.
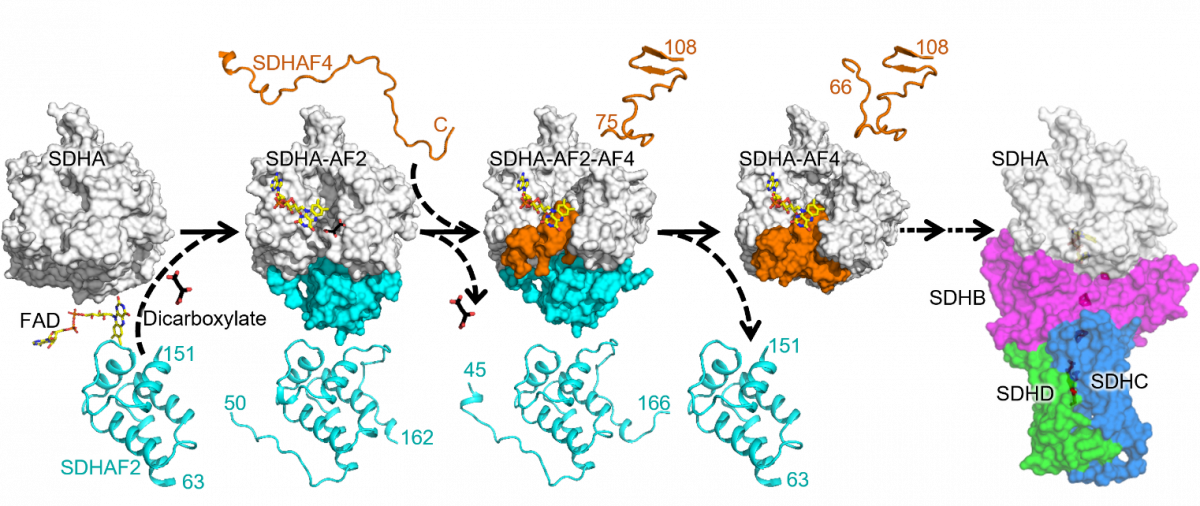
We show here that the assembly factors SDHAF2 and SDHAF4 contain intrinsically disordered regions that enables them to interact, sequentially, with SDHA, after which these regions become ordered. Further, we found that mutations/deletions of the assembly factors or SDHA result in collapse of the assembly process. Once mature SDHA is formed, the next steps of CII assembly, i.e. addition of SDHB, SDHC and SDHD, aided by SDHAF1 and SDHAF3, involves spontaneous steps culminating with formation of assembled, fully functional CII. Inspired by this research, we propose that SDHA is a master regulator of CII assembly and are studying the next steps leading to assembled CII.
Once resolved, this will present the first mitochondrial respiratory complex with fully understood assembly process. As mutations in subunits of CII are classified as tumour suppressors in several types of hard-to-treat tumours, this research has also clinical context.
Author: prof. Jiří Neužil, Institute of Biotechnology of the Czech Academy of Sciences
Publication: Sharma P, Maklashina E, Voehler M, Balintova S, Dvorakova S, Kraus M, Vanova KH, Nahacka Z, Zobalova R, Boukalova S, Cunatova K, Mracek T, Ghayee HK, Pacak K, Rohlena J, Neuzil J,* Cecchini G,* Iverson T* (2024) Disordered-to-ordered transitions in assembly factors allow the complex II catalytic subunit to switch binding partners. Nat Commun 15, 473. (*joint senior authors) doi: 10.1038/s41467-023-44563-7












