Centre of Molecular Structure
Functional clustering of surface receptors of human immune cells
A new study focused on the structure and function of natural killer cell surface receptors was recently published in the prestigious journal Nature Communications. The study comes from the research teams of Dr. Ondřej Vaněk from the Department of Biochemistry, Faculty of Science, Charles University, and Dr. Jan Dohnálek from the Institute of Biotechnology of the Czech Academy of Sciences in the BIOCEV Center.
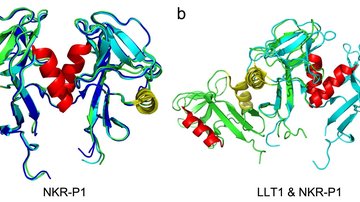
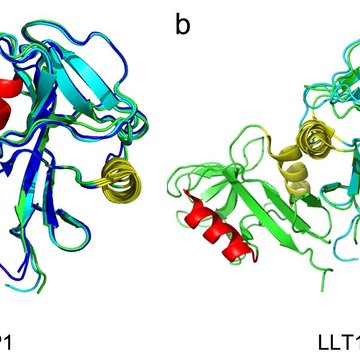
The work focuses on two proteins and their interaction: the surface receptor on NK cells called NKR-P1 and the ligand of the NKR-P1 receptor called LLT1. This protein is normally found on other cells of the immune system, and upon interaction with NK cells, they provide a signal saying that there is no need to trigger the immune response of the NK cell. However, the last fifteen years of research have evidenced that in many cases of cancer, the LLT1 protein is expressed on the surface of cancer cells, where it serves to inhibit the immune response.
The X-ray structure of the complex between the two proteins suggested the possibility that they could interact in a zipper-like structure (see illustration), helping the weak molecule-to-molecule interaction produce a more pronounced signal via aggregation of the receptors on the cell surface and so cause the inhibitory signaling to the NK cell. This hypothesis was not entirely new, but our data provided the first atomic-level clue on how such an organization could happen. It was, however, necessary to confirm the biological relevance of the structural results. This was done in the next, rather complex, steps of the research - super-resolution microscopy enabling us to observe the receptor molecules on the cell surface and functional tests with live NK cells isolated from donor blood. By combining several methods, the research team verified previous observations in the crystal structure of the complex of both proteins and described the resulting functional consequences - under what conditions the NKR-P1 and LLT1 proteins must meet to produce an inhibitory signal.
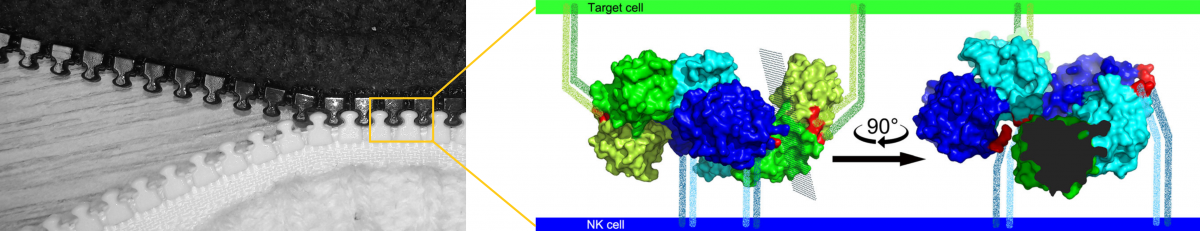
The necessity for multiple molecules to meet is thus a kind of evolutionary protection against unnecessary or false stimuli, and thanks to the new study, we can see exactly how this interaction works at the structural level. This may help design therapeutic proteins that could desirably influence the interaction between the immune system and cancer cells.
The project was realized under the lead of the team of Dr. O. Vaněk of the Laboratory of Structural Biochemistry of Immune Recognition at Charles University, with major contributions from Dr. Jan Bláha, who prepared samples, crystallized the proteins, and performed computational analysis of structural data and from Dr. Barbora Kalousková who realized the super-resolution microscopy experiments and NK cell functional assays.

The team of Dr. Jan Dohnálek of the Laboratory of Structure and Function of Biomolecules at IBT contributed by the solution of the crystal structures and their analysis and by analysis of the small angle X-ray scattering data. The major part of the structural work at IBT was done by Dr. Tereza Skálová.
Link to the original study:
Read also the article about the success of Czech scientists on the website of the Faculty of Science of Charles University - From the atom to NK cell: the story of an unexpected protein structure