Scientists reveal how leukemia cells escape therapy by rewiring their defense against protein oxidation
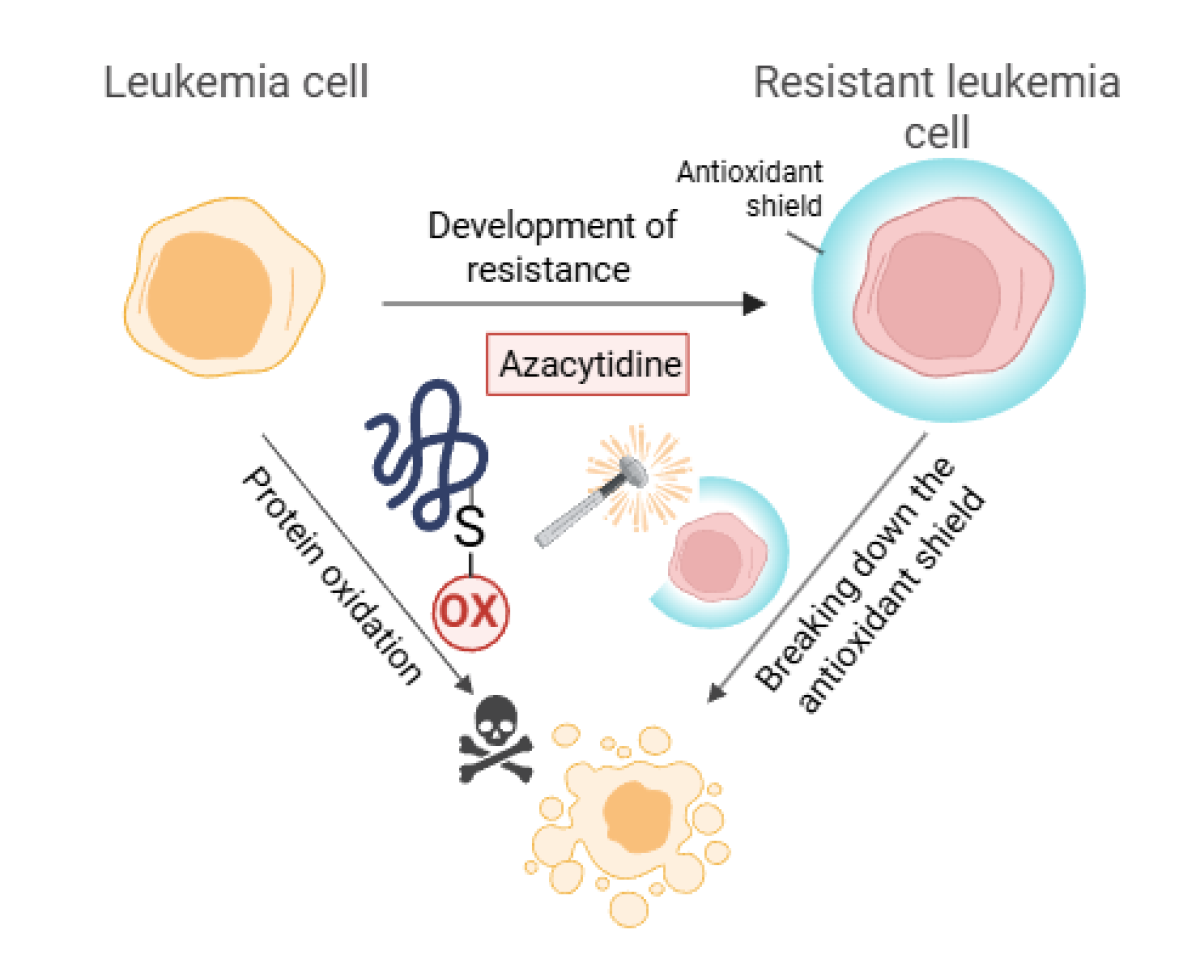
Scientists from the First Faculty of Medicine, Charles University, and the General University Hospital in Prague have uncovered why the drug azacitidine, used to treat acute myeloid leukemia, gradually stops working in patients. Their study shows that azacitidine kills leukemia cells by inducing protein oxidation, while resistance to the drug arises through the boosting of antioxidant defense mechanisms. The study was published in the journal Redox Biology at the end of 2025 and may help improve treatment strategies for this deadly form of leukemia.
Acute myeloid leukemia (AML) is a severe type of blood cancer. Azacitidine, a drug originally developed at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, is the treatment of choice for patients with AML. However, many patients eventually stop responding to therapy, and the disease relapses. Treatment options for these patients are very limited. The mechanisms underlying azacitidine’s effects and the development of resistance remain an active area of research.
In general, anticancer therapies disrupt the internal environment of cancer cells. In response, cells experience stress and begin to produce reactive oxygen species, which damage essential cellular components such as DNA, lipids, and proteins—the building blocks of cellular processes. Importantly, protein oxidation not only causes damage but can also function, at regulated levels, as a molecular switch that controls signaling pathways governing cell survival and death.
Dr. Kristýna Gloc Pimková, a scientist at BIOCEV, First Faculty of Medicine, Charles University, and head of the research group, studies how protein oxidation regulates fundamental biological processes and how leukemia cells exploit oxidative mechanisms to escape drug toxicity.
“We know that protein oxidation is one of the earliest responses of leukemia cells to drug exposure,” explained Dr. Pimková. “This regulatory layer may help cancer cells adapt and survive under therapeutic pressure.” In their latest publication, the team focused on how leukemia cells gradually adapt to evade the toxic effects of azacitidine.
“We were surprised by how critical protein oxidation is for the drug’s efficacy,” said Dušan Nemes, a PhD student and first author of the study. “Even more surprising was the finding that azacitidine selectively oxidizes only specific groups of proteins that are localized in close proximity within the cell.”
Leukemia cells escape therapy by reprogramming oxidative metabolism
The researchers also found that resistant leukemia cells behave very differently. These cells protect themselves by producing high levels of glutathione, a natural antioxidant that functions as an antioxidant shield. This shield prevents the oxidation of key proteins, thereby allowing cancer cells to survive azacitidine treatment.
“Our results show that leukemia cells can escape therapy by reprogramming oxidative metabolism, not only by acquiring genetic mutations,” Nemes added. “Most importantly, when we pharmacologically reduced glutathione levels—that is, disrupted the antioxidant shield—the resistant leukemia cells became sensitive to azacitidine again. This suggests that targeting antioxidant defenses could restore the efficacy of existing drugs.”
The findings indicate that combining azacitidine with therapies that disrupt antioxidant protection may help prevent or overcome drug resistance in leukemia patients.
The research project also involved a team from the First Department of Internal Medicine – Department of Hematology, First Faculty of Medicine, Charles University, and the General University Hospital in Prague, as part of a collaborative project funded by the Czech Science Foundation (GAČR). Another critical source of support was the National Insitute for Cancer Research, EXCELLES program – funded by the European Union.
“This study is crucial because it identifies a mechanism of resistance to azacitidine,” commented Professor Tomáš Stopka, hematologist representing the clinical team. “This opens a relatively broad avenue for validating newly identified biomarkers in clinical practice. If our assumptions are correct, it may be possible to extend the effectiveness of AML therapy, which would have a direct and practical impact on the outcomes of our hemato-oncology patients.”
Link to the publication: https://doi.org/10.1016/j.redox.2025.103958