
Scientists Identify Cells Driving Inflammation in Multiple Sclerosis
An unusual type of brain cell may play a key role in progressive multiple sclerosis (MS), likely contributing to the persistent inflammation that characterizes this disease. The discovery by an international team of experts opens a promising new path toward developing effective therapies for this neurodegenerative disorder. The study, published in the prestigious journal Neuron, involved researchers from the Institute of Biotechnology of the Czech Academy of Sciences (IBT) at the BIOCEV center.
Multiple sclerosis (MS) is a chronic disease in which the body’s immune system attacks the brain and spinal cord. A important proportion of patients gradually transition into the progressive stage of MS, characterized by continuous neurological decline and limited treatment options.
To better understand what happens during the disease, researchers collected skin cells from patients with progressive MS and reprogrammed them into induced neural stem cells. Using a “disease-in-a-dish” model, they found that a portion of the cultured brain cells reverted to an earlier developmental stage, transforming into an unusual cell type. These cells appeared approximately six times more frequently in patients with progressive MS and were named DARG (disease-associated RG-like cells).
Cells That Actively Spread Damage
The newly identified DARG cells exhibit a distinctive epigenetic profile that contributes to an exaggerated response to the immune system’s “alarm signals” (interferons), which may explain the high level of inflammation in MS.
“Progressive multiple sclerosis is a devastating condition, and effective treatments remain elusive. Our research uncovered a previously unknown cellular mechanism that appears to be central to the chronic inflammation and neurodegeneration driving the progressive stage of the disease,” said Stefano Pluchino from the Department of Clinical Neurosciences at the University of Cambridge, one of the study’s lead authors. “Essentially, we have discovered glial cells that not only malfunction but actively spread damage. They release inflammatory signals that force neighboring brain cells into premature aging, thereby sustaining a toxic environment that accelerates neurodegeneration.”
Brain Mapping by Czech Scientists
To confirm these findings, the team collaborated with researchers from the Institute of Biotechnology of the Czech Academy of Sciences, who performed an extensive re-analysis of three publicly available spatial transcriptomics datasets from postmortem human brain tissue. The analysis included more than 200 tissue sections from 31 patients with MS and 12 control individuals.
This advanced technique, which maps gene activity within the physical structure of tissue, revealed that DARG cells are not randomly distributed but are significantly concentrated in the most damaged brain regions of MS patients, particularly at the edges of chronically active lesions.
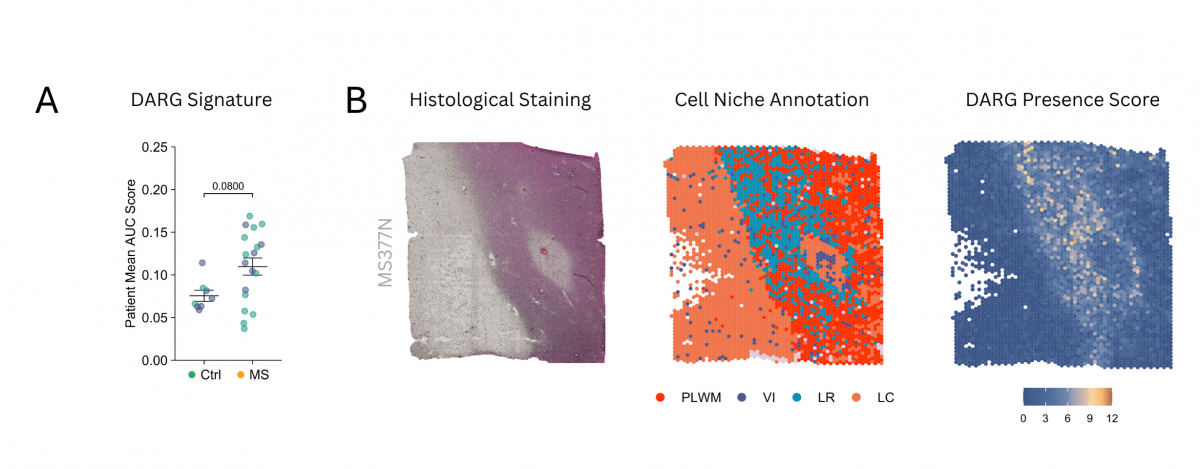
“Discovering these unique cells through the ‘disease-in-a-dish’ model was a breakthrough; the next crucial step was to determine whether they exist and where they are located in the brains of patients,” explained Lukáš Valihrach, co-author of the study from the Institute of Biotechnology CAS. “Spatial transcriptomics gives us a map showing not only which cells are present but also how they are organized and interact within damaged tissue. This work also highlights the incredible value of open science. By re-analyzing publicly available data, we were able to add critical evidence confirming that these DARG cells are concentrated precisely in those areas where the disease actively causes damage.”
Daniel Žucha, a senior bioinformatician from the same institute, added: “Working with massive spatial transcriptomics datasets requires sophisticated computational approaches. Our analysis allowed us to precisely localize the DARG cell signature within these complex tissue maps. We observed a clear spatial correlation: where DARG levels were high, there were also more inflammatory glial cells and greater tissue damage. This spatial relationship strongly suggests that they are active players in the disease process, not just passive observers.”
The discovery, published in Neuron, represents a major step toward understanding the complex mechanisms that drive the disease — and offers hope for a new type of treatment.
“We are now working to explore the molecular mechanisms that regulate DARG cells and to test potential therapeutic strategies. Our goal is to develop treatments that either correct DARG cell dysfunction or eliminate them entirely. If successful, this could lead to the first truly disease-modifying therapies for progressive MS, offering hope to thousands of people living with this debilitating condition,” said Alexandra Nicaise, the study’s lead author from the Department of Clinical Neurosciences at the University of Cambridge.
To date, DARG cells have been observed in only a few other diseases, such as glioblastoma — the most aggressive brain tumor — and cerebral cavernomas, benign vascular malformations in the brain or spinal cord. However, this may simply be because scientists have not yet had the tools to identify them. Stefano Pluchino and his colleagues believe their approach will likely reveal that DARG cells play an important role in other forms of neurodegeneration as well.
This research was funded by the Medical Research Council, Wellcome Trust, National MS Society, FISM – Fondazione Italiana Sclerosi Multipla, European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), National Institute on Aging, UK Dementia Research Institute, Austrian Science Fund (FWF), UK MS Society Centre of Excellence, Bascule Charitable Trust, Czech Science Foundation, MULTIOMICS CZ, and Ferblanc Foundation.
References
Park, B. et al. *Integrated Multi-Omics Reveals Disease-Associated Radial Glia-like Cells with Epigenetically Dysregulated Interferon Response in Progressive Multiple Sclerosis. Neuron. 2025 Oct 10:S0896-6273(25)00710-X. doi: 10.1016/j.neuron.2025.09.022.
Contacts:
Lukáš Valihrach: +420 604 763 875
Daniel Žucha: +420 776 373 548
Title image: Freepik.com
