About us
The project is focused on detailed characterization of 3D structures of complexess between proteins and small molecule drugs and ligands, e.g. glutamate carboxypeptidase II (marker of prostate cancer and target molecule of imaging methods used in neurodegenerative diseases). Several members of the histone deacetylase family are studied as markers of various leukaemias and solid tumours.
Objectives:
- Structural characteristics of complexes between proteins and drugs
- Rational design of more potent drugs
- Building the expertise for design and development of biologics as imaging probes
The Laboratory of Structural Biology (LSB) was established in March 2010. We use methods of molecular, cell and structural biology to elucidate the structure-function relationship of several diagnostic/therapeutic targets.
Ongoing projects include:
1. Identification and development of novel reagents, tools and techniques target- ing human glutamate carboxypeptidase II (GCPII) and its paralogues/orthologues.
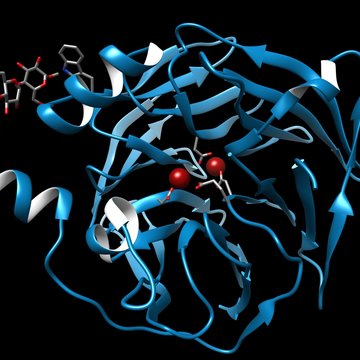
GCPII is a membrane-bound zinc metallopeptidase implicated in several physiological processes. Within the nervous system, GCPII exerts its peptidase activity by hydro- lysing N-acetyl-aspartyl-glutamate and the jejunal form of the enzyme acts as folate hydrolase, thus participating in the absorption of dietary folates. GCPII-specific inhibitors have been reported to be neuroprotective in multiple preclinical models of neurodegeneration. Additionally, given the GCPII over-expression in prostate carcino- ma and within the neovasculature of solid tumours, the protein serves as an attractive target for imaging and therapy of a variety of cancers.
Our efforts are focused on addressing questions pertaining to the fundamental (patho) physiological roles of GCPII in healthy tissues as well as in cancer and neurodegene- ration. We solved the first high-resolution X-ray structure of human GCPII and these data, together with more than 40 reported structures of GCPII-inhibitor complexes, serve as a basis for our extensive programme aimed at the structure-assisted design of novel inhibitory compounds targeting GCPII. In addition to projects involving small-molecule GCPII inhibitors we are actively pursuing the development of biolo- gics (Anticalin-based scaffolds) as novel agents for prostate cancer imaging. In our basic-oriented research we aimat unravelling putative non-enzymatic role(s) of human GCPII (receptor, chaperone) and defining functions of GCPII orthologues in several model organisms, including A. thaliana and C. elegans.
2. Structure-function studies of histone deacetylases (HDACs)
Lysine acetylation is a major post-translational modification that plays a key role in many physiological processes and is believed to have a broad spectrumof modulatory functions within the cell. Acetylation levels of cellular targets are regulated by opposite activities of histone acyltransferease and histone deacetylases (HDACs). Eighteen hu- man HDACs identified so far can be divided into four major classes and our laboratory is specifically interested in class IIb (HDAC6, HDAC10) and class IV (HDAC11) pro- teins. To address basic questions related to the structure-function relationship of the- se hydrolases we employ a panel of molecular biology, biochemistry and enzymology techniques targeting the individual HDACs as well as their cognate substrates.
In addition to our own research, we actively participate in several collaborative projects that takeadvantageof aportfolioof techniques establishedinour lab.Thesetechniques include heterologous protein expression in several systems including E. coli, K. lactis, insect S2andHi5cells, andmammalianHEK293cells, enzymatic andphysicochemical characterizationof purifiedproteins andX-ray structuredetermination, includingrobotic screening of crystallization conditions.