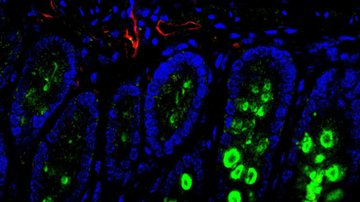
Metabolism of healthy and tumor tissue at single cell resolution
Researching the way cancer cells communicate brings hope for new treatment
Cancer cells need an immense amount of building material to proliferate. When they run out, they are able to persuade healthy nearby cells to supply them. How they do so is the subject of research done by Kateřina Rohlenová from the Institute of Biotechnology of the CAS: “We already know quite well what happens on the inside of cancer cells. We now want to map what happens in their surroundings.” The story originally appeared in Czech as “Insatiable cancer cells” in the CAS magazine A / Věda a výzkum.
I need to bring in more construction materials for my building – could you create a detour off the main lane and build a new road here, please? That’s what a tumour’s plea for more nutrients might look like. In this case, the road is a blood vessel. Cells can indeed communicate with each other in a similar way, though via molecules, not words. But the “communication” or crosstalk of cells is very sophisticated and not yet fully understood. In fact, we hardly understand it at all.
Intercellular communication also comes into play during cancer treatment. Cancer cells need to divide. But to do so, they consume a lot of the building material produced by cellular metabolism, especially metabolites called nucleotides – the basic building blocks of DNA or RNA. Cells have only two options for obtaining them: either from their external environment or they have to create them themselves.
Each cell has its own metabolism and as part of this, it produces nutrients as a byproduct. Some of these nutrients are consumed by the cell itself, others are excreted; these can then be used by another cell in the vicinity. Also at this stage, a common “language” comes into play. Certain cancer cells seem to ask for more metabolites, and others supply them on the basis of their ‘order’. How precisely this mechanism works and how it could be used in treatment is being investigated by Kateřina Rohlenová from the Institute of Biotechnology of the CAS: “We already know quite well what happens on the inside of cancer cells. We now want to map what happens in their surroundings.”
A brief look at the history
The idea that faster growth could be used to treat cancer is not new. On the contrary, this is the principle upon which chemotherapy has worked from the very beginning. To clarify: the term “chemotherapy” is an umbrella term referring to any treatment of any disease by means of a synthetic substance (for instance, even treating a headache with ibuprofen), whereas cancer chemotherapy uses so-called cytostatic drugs – substances that are toxic to cells. In short, they stop cell growth, cell division. Unfortunately, this applies not only to cancer cells, but across the board.
Let’s take a brief look at the history first. The first attempts at chemotherapeutic treatment of cancer occurred during the Second World War, when doctors noticed a loss of white blood cells in sailors in Italy affected by yperite. A breakthrough in the treatment of metastatic cancers came with methotrexate. First prepared in 1947, the chemotherapy agent has been used to treat cancer since 1956 (and not only in oncology, but also in rheumatology and dermatology).
Methotrexate is a folic acid antimetabolite. Simply put, it prevents the formation of a substance that is necessary for the formation of DNA. In 1957, researchers discovered fluorouracil – a purine antimetabolite (i.e., the very building blocks of DNA and RNA). This substance is also still used to this day.
Mapping the metabolic crosstalk
If cancer cells are prevented from making more DNA or RNA by, say, the two pharmaceuticals just mentioned, they cannot divide and produce proteins and tumour growth therefore stops. So what’s the catch? (If there were none, we would be able to treat cancer as successfully as we treat, for instance, tonsillitis). Cancer cells have the ability to evade the treatment, and they develop a resistance to it. Why? We’re not sure. “We’re trying to find out how this happens in the natural environment of tumours. Because in a tumour, cancer cells are never found alone. They interact with healthy tissue,” Rohlenová says, explaining the focus of her research. “Although the word ‘healthy’ is not accurate in this context. Even non-cancerous cells inside a tumour behave differently from normal cells of the same tissue elsewhere. We just aren’t exactly sure how. And that’s what we want to find out.”
New methods developed in recent years have opened up the opportunity for this research. It is now possible to study metabolic communication down to the level of individual cells. At the same time, however, the data can be combined with another type of laboratory procedure, which instead focuses on the distribution of cells in the tumour. In an ideal case, the result would comprise a kind of map of how the cells behave and communicate, how their own metabolism is set up, how they use their own or other cells’ building blocks, and then one day, an effective treatment could be sought based on these findings. “But we’re in this for the long run and currently only at the beginning. The goal may still be ten or fifteen years away,” says Rohlenová.
The researcher has plenty of insight into the issue, having worked as a PhD student in the team of Jiří Neužil, also from the Institute of Biotechnology of the CAS, where her focus was on breast cancer. More specifically, she was researching the mechanism of action of the anti-cancer compound MitoTam, which recently successfully passed the first phase of clinical trials. The process has taken approximately a decade since the discovery of the substance. “I continue to follow its fate, and I recently managed to publish a report on the results of the first phase of trials, where I figure as a co-author as well. But at this point, MitoTam is not so much about research per se as it is about negotiating with investors,” Rohlenová remarks.
However, Rohlenová was interested in doing her own research. After returning from a postdoctoral stint in Belgium, she established the Laboratory of Cellular Metabolism at the Institute of Biotechnology of the CAS, located – like the entire institute – on the premises of the BIOCEV Centre in Vestec, just outside of Prague. At first, it was a smallscale endeavour, with a team of two students and her husband Jakub, an expert in tumour metabolism. And to make matters more challenging – “I founded the lab while pregnant,” she reveals. Today, she is working there full-time and has been awarded a prestigious ERC Starting Grant. It is not only thanks to this grant that her lab has now expanded to 12 employees.
Back to the Czech Republic
Not every promising, or even successful, Czech researcher returns to their homeland after working abroad. Fortunately, the trend has slowly been turning around lately. Kateřina Rohlenová is one example of this. She was attracted back to the Czech Republic not only by the opportunity to put together her own research team and lab, but also by the research conditions she has at her disposal at the BIOCEV Centre. The Czech Centre for Phenogenomics, which is a part of BIOCEV, was another reason. “It is a top-notch workplace in the international context as well,” Rohlenová adds.
It is there that they prepare the unique mouse models that her Laboratory of Cellular Metabolism needs for its research. The principle is as follows. It is necessary to find out where cancer cells obtain the building blocks for their division (i.e., the aforementioned metabolites) if they do not have or cannot manage to produce them themselves. One of the experiments Rohlenová is planning to do is to create a situation in which the cells of a mouse model produce metabolites, in this case nucleotides, and the researchers populate the model with cancer cells whose own nucleotide production is switched off. They must therefore rely solely on an external source.
Another thing is also needed: a special mouse model that is unable to produce nucleotides. How do normal cancer cells adapt to such an environment then – how do they cope with the lack of building material? “Basically, we are simulating the effects of systemic antimetabolite treatment and figuring out its direct effects not only on cancer cells, but also on other cells in the tumour environment that are capable of producing nucleotides. Our models will allow us to examine how the tumour responds to the selective switching off of synthesis in cancer cells and beyond them. This will also allow us to test various mechanisms of resistance,” the researcher explains the ambitious project.
A far-off target
Using similar experiments, Rohlenová wants to map metabolic crosstalk. If, for instance, a cancer cell has a reduced ability to produce nucleotides, what are the potential donors that help it out? If researchers figure this out, they will be able to target not only cancer cells but also their ‘helpers’ during treatment.
“By getting down to the level of individual cells, we will know exactly what each one expresses. Then if we were to discover something – a gene, a protein – that is specific to cancer cells, that would be a win and we could target the treatment directly at them,” Rohlenová explains. “However, this is very unlikely to happen.” Achieving the goal will therefore be a more difficult endeavour.
Indeed, outside the field of metabolism, similar approaches to treatment already exist, but they are not very successful. Even when the treatment is targeted, cancer cells find their own ways to circumvent the therapy. So we need to figure out how the cells adapt to the treatment and the lack of nutrients, what pathways they then begin to use... The situation is complicated by the fact that cancer cells in the tumour are not uniform, homogeneous. That is why we need to obtain the aforementioned map.
For the time being, the cancer cells have a head start. It’s as if they have their own map and are constantly redrawing it to suit their own needs. Every now and then they build a new road or convince cells in the vicinity to start producing the “material” they currently need. Rohlenová is examining how they “talk” to each other. So far, the tumours seem to be very convincing in their communication.