Genomics of Eukaryotes and Lateral Gene Transfer
An Interesting Collaboration Revealed
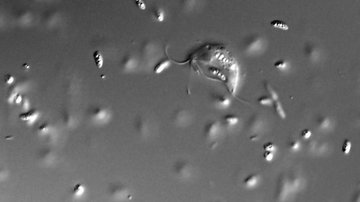
In a new study, researchers from the Faculty of Science of Charles University and BIOCEV, in collaboration with researchers from Max-Planck Institute for Terrestrial Microbiology and European Molecular Biology Laboratory from Heidelberg, have delved into the intricate partnership between anaerobic amoebae from the genus Pelomyxa and their prokaryotic companions. Pelomyxa, known for their unique symbiotic relationships with multiple prokaryotic endosymbionts, has long mystified scientists regarding the role these symbionts play in their host's metabolism.
Using single-cell genomics and transcriptomics, the research team has unraveled the hidden secrets of the prokaryotic community thriving inside Pelomyxa schiedti. This symbiotic consortium is comprised of two distinct bacteria, Candidatus Syntrophus pelomyxae (class Deltaproteobacteria) and Candidatus Vesiculincola pelomyxae (class Clostridia), along with a methanogen known as Candidatus Methanoregula pelomyxae.
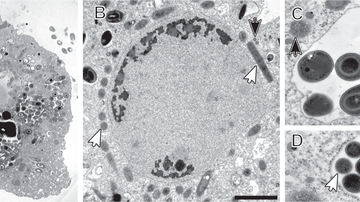
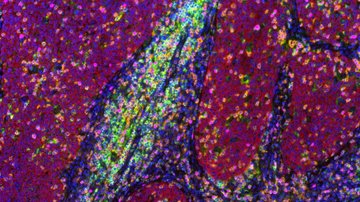
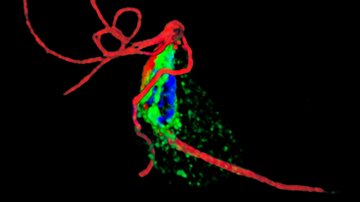
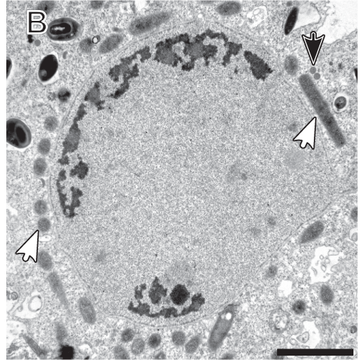
The study, recently published in The ISME Journal, sheds light on the spatial distribution of these symbionts within the host amoeba. Candidatus Vesiculincola pelomyxae was found to be localized within vesicles, while the other endosymbionts were freely distributed in the cytosol. Interestingly, Candidatus Methanoregula pelomyxae was enriched around the nucleus.
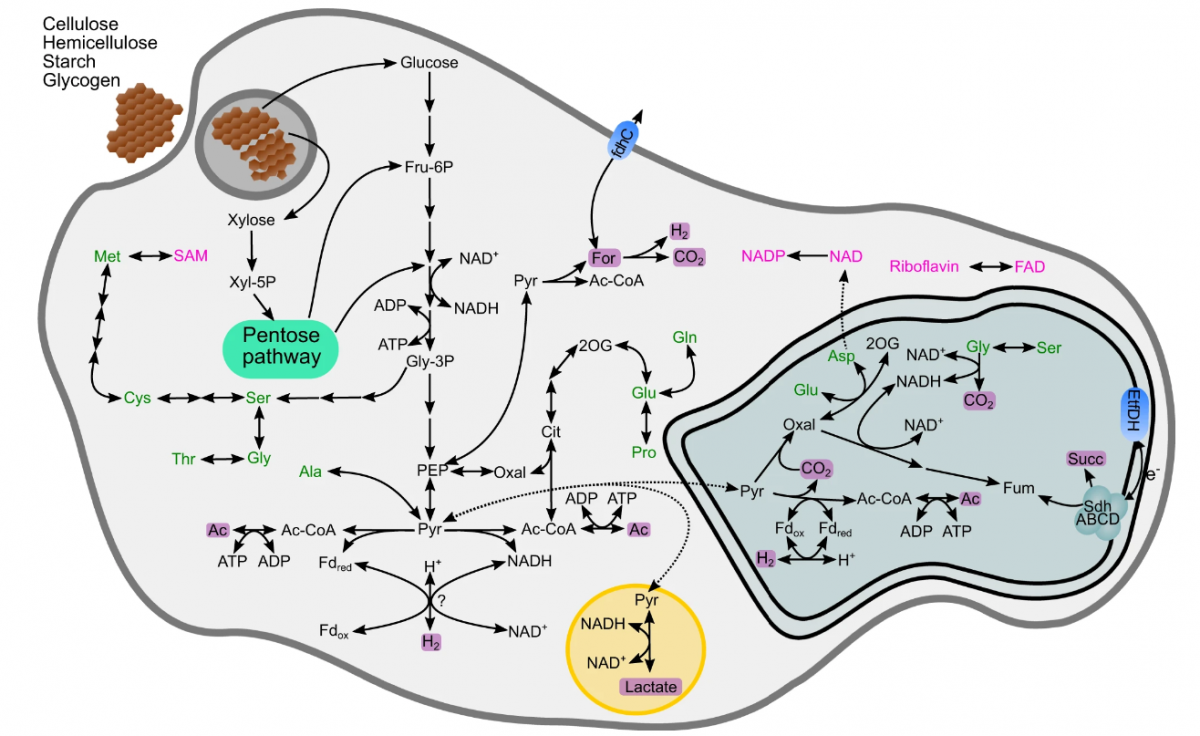
An in-depth analysis of the genomes and transcriptomes of these symbiotic partners revealed their metabolic strategies. The cellulolytic activity of Pelomyxa schiedti is expected to produce simple sugars that serve as fuel for both its own metabolism and Candidatus Vesiculincola pelomyxae. On the other hand, the energy metabolism of Candidatus Syntrophus pelomyxae's relies on the degradation of butyrate and isovalerate from the environment. Notably, both bacteria and the amoeba employed hydrogenases to facilitate the transfer of electrons, a process dependent on low hydrogen partial pressure. This crucial regulation was achieved by Candidatus Methanoregula pelomyxae, which utilized H2 and formate for methanogenesis.
The researchers conducted experiments to understand the indispensability of these symbiotic interactions. While the bacterial symbionts were successfully eliminated by vancomycin treatment without affecting the viability of the amoebae, the use of 2-bromoethanesulfonate, a specific inhibitor of methanogenesis, proved fatal for the amoebae. This underscored the central role of methanogenesis in the survival of this intricate consortium.
Commenting on the results, Dr Sebastian Treitli, a member of the research team and first author of the study, said: "This study reveals the fascinating strategies employed by these symbiotic organisms to thrive together. While we know of many symbioses between eukaryotes and archaeal methanogens, this is the first example where the methanogenic symbiont is obligate. We are almost certain that similar metabolic couplings with a central role for methanogenesis are very common in anaerobic environments, only the actors are changing..."
This research not only adds to our knowledge of symbiosis, but also holds potential implications for fields ranging from microbiology to ecological studies. The study illustrates the intricate and interconnected nature of life forms and provides a deeper understanding of the delicate balance that sustains ecosystems.
Title image: Transmission electron micrographs of Pelomyxa schiedti cells. - Link to Fig. HERE
Citation:
Treitli, S.C., Hanousková, P., Beneš, V. et al. Hydrogenotrophic methanogenesis is the key process in the obligately syntrophic consortium of the anaerobic ameba Pelomyxa schiedti. ISME J (2023). https://doi.org/10.1038/s41396-023-01499-6