About us
This project is focused on the optimization of DNA immunization against tumors induced with human papillomaviruses (HPVs). To enhance the efficacy of this antitumor vaccination, various fusion genes that express antigens with favorable characteristics (e.g. with inserted strong universal helper epitopes or with cellular localization that targets antigens into pathways of MHC class I and/or class II presentation) are constructed. Furthermore, the research team attempts to augment the antitumor effect of DNA immunization by combination with other immunotherapeutic approaches – blockade of immune checkpoint receptors with monoclonal antibodies and activation of toll-like receptors with their ligands.
Murine lung metastases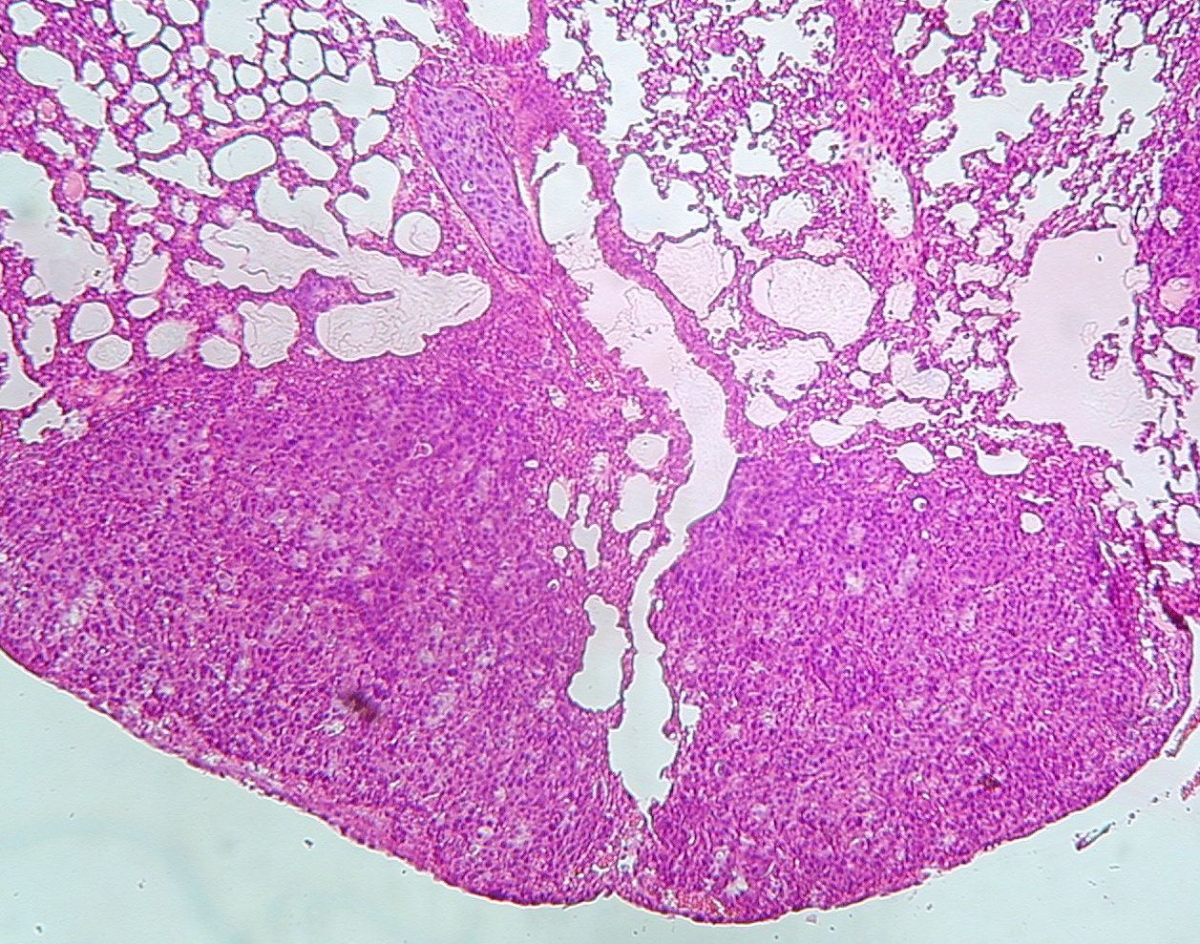
DNA vaccines that are delivered by a gene gun are aimed against the viral oncoprotein E7, which represents a tumor specific antigen. The efficacy of immunotherapy is examined in mouse tumor models that are also developed in the laboratory, including derivation of tumor cell lines with reduced expression of MHC class I molecules. As this reduction is one of the most frequent mechanisms of tumor escape from the host immunity, it could contribute to the limited therapeutic effect of cancer immunotherapy in clinical trials.
To study factors that influence the effect of cancer immunotherapy, mouse oncogenic cell lines are modified with the CRISPR/Cas9 system and immune reactions induced by immunotherapy are analyzed. Particularly, immune cells that infiltrate tumors with various MHC class I expression and contribute to an anti-tumor effect are characterized. Moreover, spatial and temporal heterogeneity of immune cells in tumor microenvironment is analyzed. Finally, the interaction of some human oncogenic viruses with plasmacytoid dendritic cells is investigated.
Potential for cooperation
In this project, we can cooperate with research laboratories studying tumor immunology, immunotherapy of malignant diseases, and tumor escape mechanisms. We also welcome cooperation with clinical institutions that are interested in immune reactions in patients with malignancies or that are performing clinical trials of cancer immunotherapy. Next, we can cooperate with pharmacological companies in preclinical testing of combined antitumor therapy that includes immunotherapy, especially immune checkpoint blockade. In these studies, we can provide our mouse models of tumors with downregulated MHC class I expression and analyze immune reactions by ELISPOT and ELISA assays, immunohistochemical staining of tumors, and flow cytometric phenotyping of tumor-infiltrating immune cells.