Mechanisms for Transport of Proteins through Mitochondrial and Bacterial Membranes
Can mitochondria export proteins to the cytosol? They most likely can.
Pavel Doležal's team from the Faculty of Science, Charles University in the BIOCEV centre has identified a mitochondrial pathway derived from the bacterial type II secretory system (T2SS) in several eukaryotic lines. This pathway is known for its ability to transport packaged proteins and protein complexes across the outer bacterial membrane. The results were published in the journal Nature Communications.
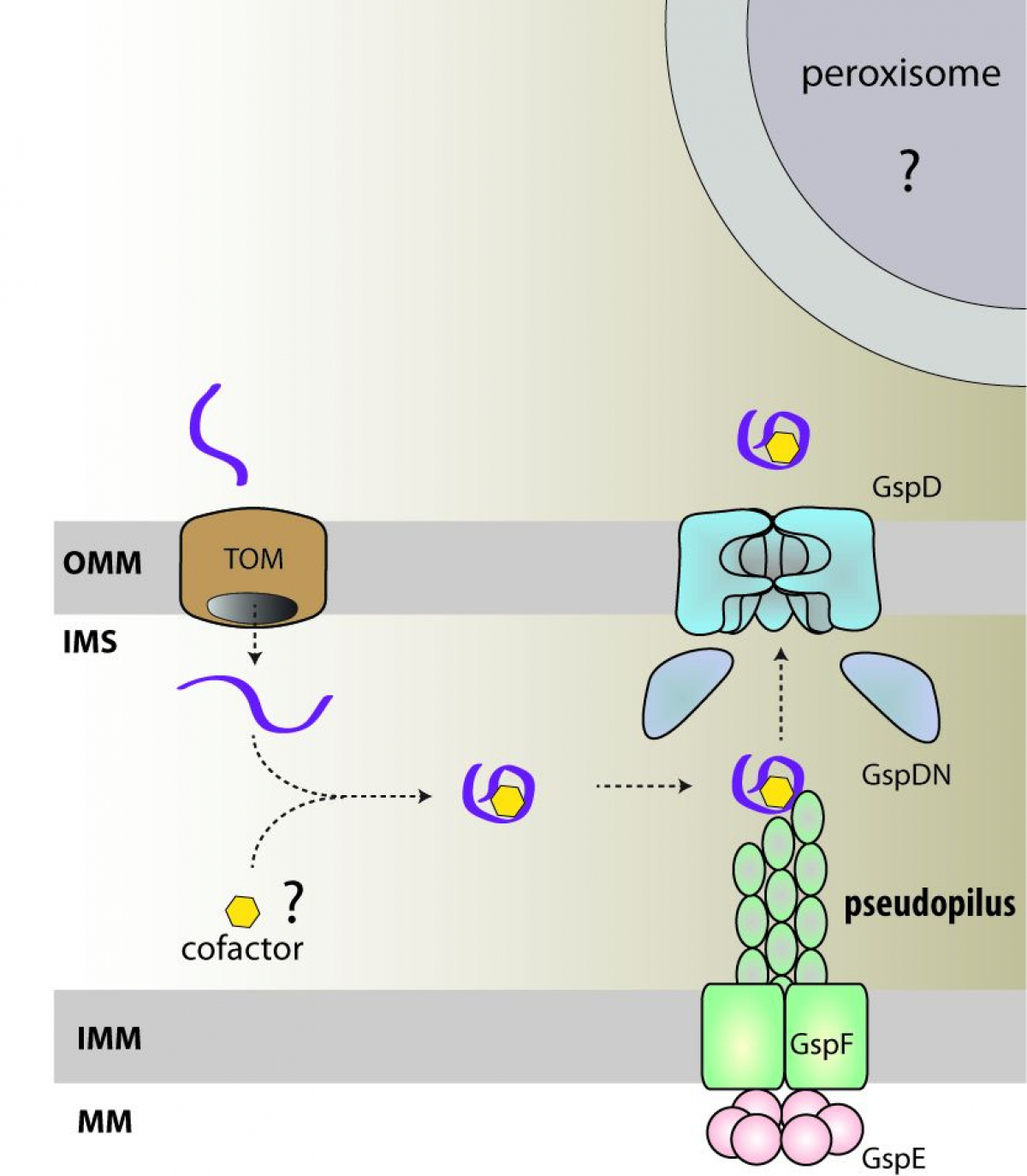
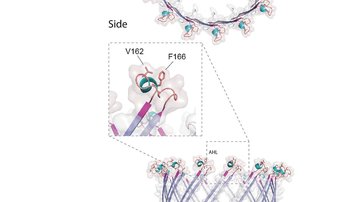
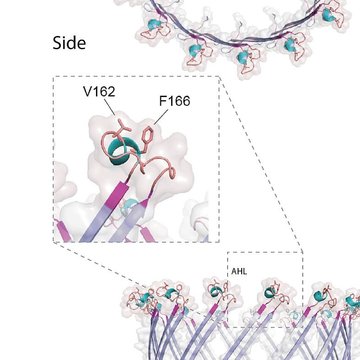
"Free-living and pathogenic gram-negative bacteria secrete enzymes and toxins into their surroundings. One of the main pathways of this secretion is T2SS, which expels proteins across the outer membrane through the secretory pore by the polymerization of pseudopil," says Pavel Doležal, the main author of the publication and adds: "Genomic analyses of several single-celled eukaryotic lines revealed the presence of four key T2SS subunits. These four subunits of a bacterial origin are complemented by twenty eukaryotic proteins, which form a unique system that likely combine the functions of mitochondria and peroxisomes."
The initial functional characterization of the four basic subunits indicated the presence of a large pore in the outer mitochondrial membrane, through which proteins are most likely transported from the intermembrane space to the cytosol and further to peroxisomes. Mitochondrial T2SS was present in the last common ancestor of all eukaryotes and was lost or replaced by another mechanism in most groups.
"The next part of the project will therefore focus mainly on the identification of substrates that are exported from mitochondria. Their function could reveal the essence of the original interaction between the mitochondria ancestor and the host cell,” Pavel Doležal concludes.
See full article of Doležal Lab in Nature Communication HERE