About us
The project will focus on deciphering the function of proteasomal shuttling proteins, particulary those connected to DNA damage. For selected proteins we will prepare knock-out mice and analyze their phenotype.
Research Areas:
- Ubiquitin-proteasome pathway
- Ddi1-like proteins
- Hepatitis B virus
Objectives:
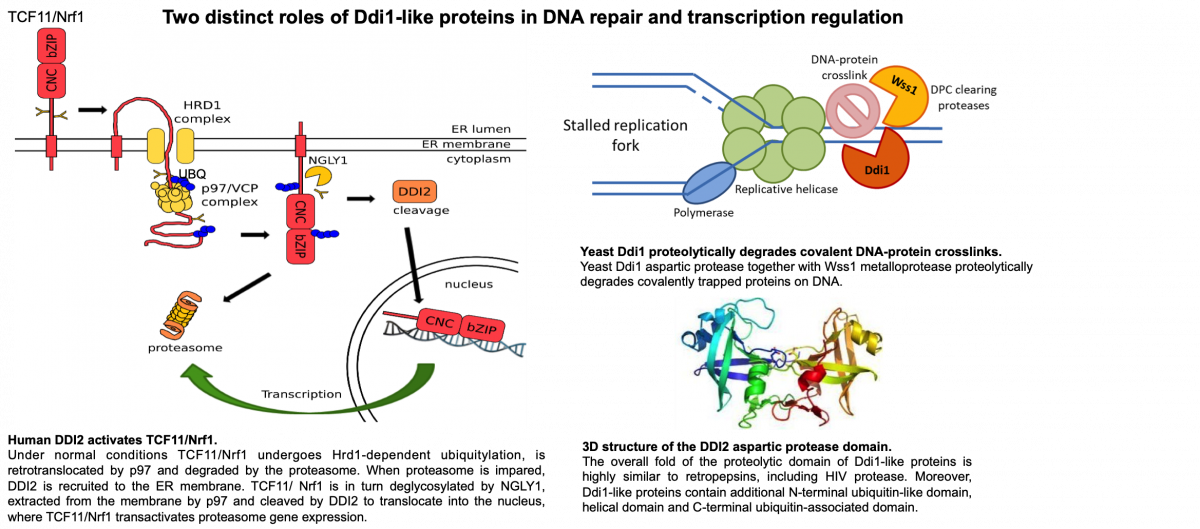
- Ddi1-like proteins in DNA repair and transcription regulation
- Development of nanoparticles for mRNA targeted delivery
- Development of antivirals targeting HBV
Content of the research:
Our junior group was established in 2016 at the research center BIOCEV. We are a proud member of the Department of Genetics and Microbiology, Faculty of Science, Charles University in Prague.
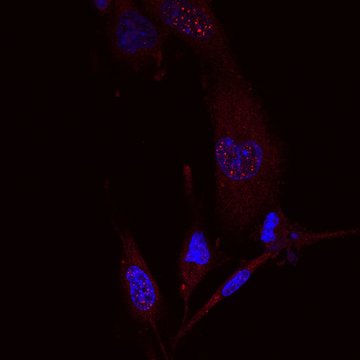
We are interested in the family of DDI1-like proteins involved in DNA repair and transcription regulation. We also focus on hepatitis B virus and work on HBV cure.
In brief, we focus on Ddi1-like proteins (DNA damage-inducible protein 1) that were largely understudied, but that have been brought up to general interest in the past couple of years. The reason is simple: Ddi1-like proteins are newly discovered players involved in DNA repair and also in transcription regulation. The complete mechanism of both Ddi1 activities however still remains to be uncovered.
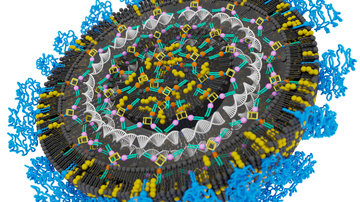
We are also interested in the biology of hepatitis B virus, particularly the covalently closed circular DNA (cccDNA), which resides in the nucleus of infected hepatocytes as a non-integrated plasmid-like molecule and serves as a transcriptional template for HBV. Its elimination is a key step towards possible HBV cure. We thus focus on various strategies for cccDNA degradation, such as development of nanoparticles transporting CRISPR/Cas9 system targeted to cccDNA.
Potential for cooperation
We are always looking for talented and motivated students and postdocs.
More information about our lab is available HERE